
What is soil pH?
Definition and implications for the garden
Contents
Soil pH, abbreviation of “potential hydrogen”, allows assessment of its acidity and fertility. Most plants thrive in soils with a pH between 6 and 7.5, close to neutrality at 7. It is indeed a balance point where all a soil’s potential can be realised. Outside this range, various problems affect plant well-being unless plants adapted to the medium are chosen!
What does pH measure?
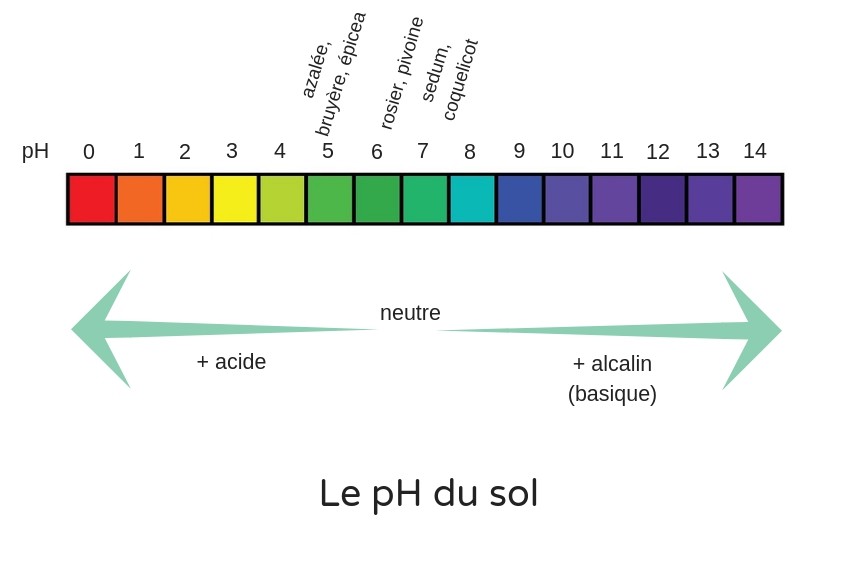
pH or hydrogen potential is an index between 0 and 14 that allows you to know the acidic or alkaline (calcareous) character of your soil. It measures activity of hydrogen ion H+ in a solution.
pH is measured on a scale from 0 to 14 :
- 0 is maximum acidity: very high concentration of acids (and very low concentration of bases)
- 14 is maximum basicity or alkalinity: very low concentration of acids (and very high concentration of bases)
- and 7 neutrality (equivalent concentration of acids and bases).
Why measure pH?
Knowing this chemical parameter helps explain why some plants do not grow well despite your care:
- A pH below 5 limits growth of most plants, causing symptoms of calcium deficiency (Ca) except in plants known as « calcifuge » or « heather soil » plants (Rhododendrons, heathers, azaleas…). This type of soil is very poor and often toxic for plants because of aluminium release. Phosphorus (P) becomes fixed and therefore unavailable, which is why adding phosphorus in the form of bone meal, feather meal or phosphate slags is strongly recommended in very acidic soil to stimulate root growth.
- A pH above 8 also limits plant growth because plant struggles to absorb soil nutrients, notably iron and magnesium. This shows as yellowing of leaves between veins, called iron chlorosis.
If pH of your soil tends towards these extremes, you therefore have the option:
- to choose plantings suited to those conditions, especially if its value falls outside range 6 to 7.5, which suits most plants.
- to correct pH with varying success depending on case (see below).
To assess its pH without using a soil analysis laboratory, see our factsheet “How to measure soil pH“.
A map of French forest soils and observation of wild (or cultivated) plants can help confirm your measurement.
Which criteria might alert you?
Poor growth of plants can reveal a pH-related problem :
- Vegetable garden plants of the Legume family as well as those of the Brassicaceae dislike very acidic soils. They show various symptoms such as low yields (peas, beans, cabbages, beetroot), diseases especially in alfalfa, a hollow, hard root in turnips… Potato, on the other hand, does well in acidic soils (pH 6).
- Slow, difficult decomposition of your plant waste on soil indicates low microbial activity generally due to excess acidity. This type of very acidic soil (pH 5) is ideal for establishing the ericaceous plants : Japanese maples, rhododendrons, azaleas, camellias, heathers…
- Foliage that yellows, particularly between the veins (iron chlorosis) indicates that your soil is very calcareous. This yellowing affects photosynthesis and consequently growth of these plants which are intolerant of active lime. There are many pH-indifferent or calcicolous plants that may suit : field maples, cistus, rosemary, lavender, legumes, Brassicaceae…

Typical sign of iron chlorosis
Can soil pH be modified?
- Liming — that is the addition of lime is an ancient technique that allows raising the pH of very acidic soils. It enables proper functioning of the clay-humus complex, that association of clay and humus capable of storing and making available to the plant essential mineral elements.
- Lowering pH is more complicated, at least in the long term, because even if you create a pocket filled with turf, potting compost or garden compost, the roots will eventually overflow and come into contact with active lime (fine fraction of total lime in the soil, easily dissolved and responsible for iron chlorosis when it exceeds 6 to 10% depending on plant sensitivity).
What causes soil pH to vary?
Nature of parent rock
pH depends largely on nature of parent rock :
- Calcareous rock will give soil a pH around 8.3
- Some saline soils are even more alkaline
- Sandy or silty sedimentary rock most often produces an acidic pH (below 7), but calcareous sands also exist.
- Granite or schist subsoils generate an acidic soil
- Volcanic soils generally have pH between 4.5 and 6.5
- Peaty soils can drop to a pH of 3.5…
Humification of organic matter
Soil naturally tends to acidify in natural or cultivated medium due to humification of organic matter. Addition of acidic fertilisers (ammonium) further accentuates this phenomenon if soil is not ploughed. Growing legumes (peas, beans, lucerne…) slowly acidifies soil at surface and depth, mainly by releasing ammonium NH4+.

Legumes, such as lucerne, acidify soil
Season
pH is higher in winter because H+ ions are less concentrated in soil solution due to higher moisture. In summer, production of organic acids caused by increased biological activity in this season and lower soil water content reduce pH. These seasonal variations are on the order of a few tenths of a pH unit, but can reach 0.5 to 1 unit in calcareous soils.
- Subscribe!
- Contents
































Comments